Pfizer asks for formal US approval of oral COVID treatment Paxlovid
(VOVWORLD) - Pfizer said on Thursday it is seeking full U.S. approval for its oral COVID-19 antiviral treatment Paxlovid, which is currently available under an emergency use authorization (EUA).
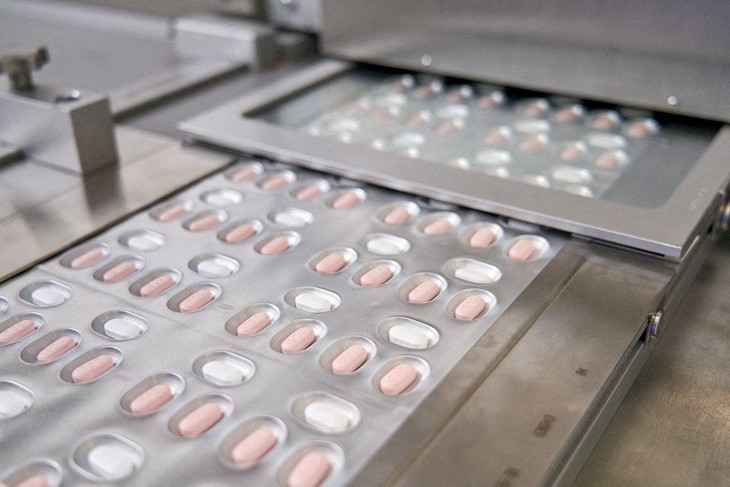 Pfizer's coronavirus disease (COVID-19) pill Paxlovid is packaged in Ascoli, Italy, in this undated image obtained by Reuters on November 16, 2021. (Photo: REUTERS) Pfizer's coronavirus disease (COVID-19) pill Paxlovid is packaged in Ascoli, Italy, in this undated image obtained by Reuters on November 16, 2021. (Photo: REUTERS) |
Pfizer said it submitted a New Drug Application for Paxlovid to the Food and Drug Administration for the treatment of COVID-19 in vaccinated and unvaccinated people at high risk for progression to severe illness.
Data from a study in Israel earlier this month showed Paxlovid reduced COVID-19 hospitalization and death rates in vaccinated and unvaccinated patients 65 years and older, but was not found to prevent severe illness among younger adults.