(VOVWORLD) -The Military Medical University gave the 2nd jab of Nanocovax, a COVID-19 vaccine developed by Vietnam, to volunteers on Wednesday.
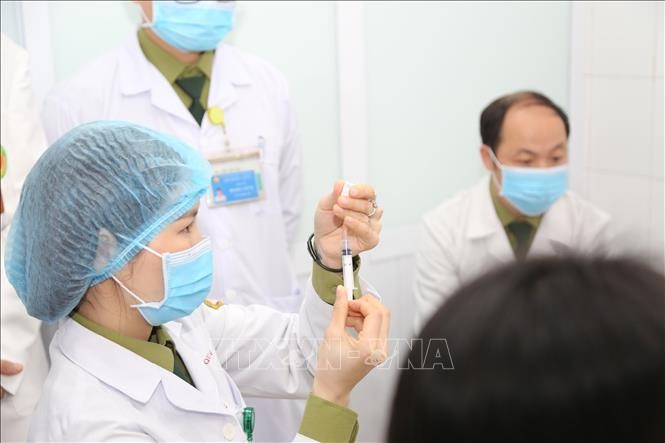 Doctors prepare to inject the 50 mcg dose Nanocovax vaccine for volunteers. Doctors prepare to inject the 50 mcg dose Nanocovax vaccine for volunteers. (Photo: VNA)
|
Among them, three people, who had been injected a dose on December 26, 2020, were given a dose of 50mcg and the remainders got a dose of 25 mcg. Their health conditions remain stable, marking the half way through for the locally made vaccine during the first stage of human trials regarding its safety.
Associate Prof. Ho Anh Son, Deputy Director of the Military Medical University’s Institute of Biomedicine and Pharmacy,said the test results 7, 14 and 28 days after the injection showed that the vaccine is capable of producing immunity and ensuring safety.
Nanocovax is developed by the Nanogen Pharmaceutical Biotechnology JSC, one of four organisations researching COVID-19 vaccines in Vietnam, is scheduled to be tested on 60 selected volunteers aged between 18 and 50 in the first phase of human trials. Each volunteer will receive two shots of the vaccine with a gap of 28 days.
Stage two proposal documents will be submitted to the Health Ministry next month.