Vietnam’s vaccine regulation system reaches WHO’s 2nd highest ranking
(VOVWORLD) -The World Health Organization has formally recognized Viet Nam’s National Regulatory Authority as achieving Level 3, the second highest level in the WHO’s four-level NRA rating scale.
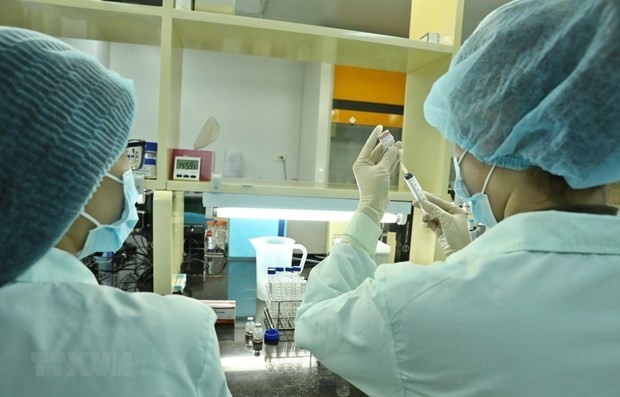 Staff of Vaccine and Biological Products No. 1 Company (VABIOTECH) testing the amount of protein in the COVID-19 vaccine. Photo: Minh Quyet / VNA Staff of Vaccine and Biological Products No. 1 Company (VABIOTECH) testing the amount of protein in the COVID-19 vaccine. Photo: Minh Quyet / VNA
|
The NRA assessment was conducted by using the Global Benchmarking Tool (GBT) of WHO to ensure the quality, safety, and effectiveness of vaccines.
The National Regulatory Authority is compliant in all areas required to provide regulatory oversight of vaccines: overall system framework; marketing authorization and licensing; post-marketing surveillance, including for adverse events following immunization; lot release; laboratory access; regulatory inspections of manufacturing sites and distribution channels; and authorization and monitoring of clinical trials.
Vietnam is now capable of manufacturing 11 vaccines against 11 diseases for the national expanded immunization program, including tuberculosis, diphtheria, whooping cough, tetanus, hepatitis B, Japanese encephalitis, cholera, typhoid, measles, rubella, and polio, in addition to other vaccines like seasonal influenza, H5N1 and rotavirus.